The Master Batch Record Template You’ll Ever Need: A Comprehensive Guide
In the highly regulated world of pharmaceutical manufacturing, biotechnology, and food processing, meticulous record-keeping is not just best practice – it’s a legal requirement. The Master Batch Record (MBR) serves as the cornerstone of this process, acting as a blueprint for manufacturing a specific batch of a product. A well-designed MBR template ensures consistency, accuracy, and traceability, ultimately safeguarding product quality and patient safety. This article provides a comprehensive guide to creating and utilizing the master batch record template you’ll ever need, covering key elements, best practices, and frequently asked questions.
What is a Master Batch Record (MBR)?
The Master Batch Record is a detailed, step-by-step instruction manual for manufacturing a specific product. It’s a living document that meticulously outlines every aspect of the production process, from raw material sourcing and preparation to packaging and final release. Think of it as the ultimate recipe, ensuring each batch is produced consistently and in accordance with established quality standards.
Key Components of a Robust Master Batch Record Template
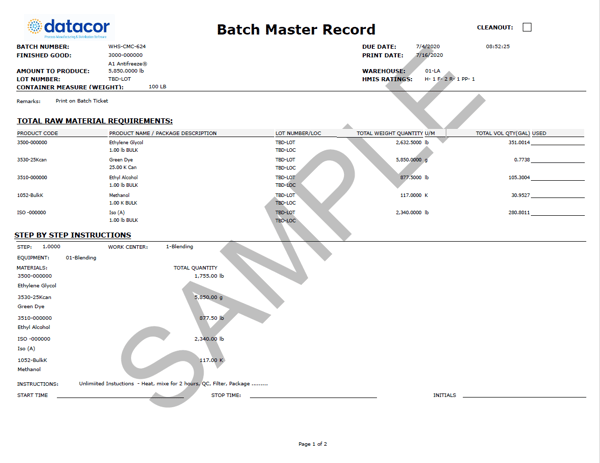
A comprehensive MBR template should include the following essential sections:
Product Information:
- Product Name and Code
- Batch Size
- Manufacturing Site
- Revision Number and Date
- Manufacturing Dates (Planned and Actual)
- Expiration Date
- Reference to the Approved Standard Operating Procedure (SOP)
Raw Material Information:
- Detailed list of all raw materials, including:
- Material Name and Code
- Supplier Information
- Lot Numbers
- Quantity Required for the batch
- Acceptance Criteria (e.g., specifications, testing methods)
- Instructions for material receipt, storage, and handling.
- Detailed list of all raw materials, including:
Equipment Information:
- List of all equipment required for each step.
- Equipment identification numbers (Unique identifiers)
- Cleaning and sanitization procedures for equipment.
- Calibration requirements.
Manufacturing Process:
- Detailed, step-by-step instructions for each stage of the manufacturing process.
- Specific instructions for each operation, including:
- Mixing times and speeds
- Temperature and pressure parameters
- Addition of materials (sequence and timing)
- Sampling procedures (locations, frequency, and methods)
- Critical Process Parameters and their acceptance criteria.
- Space for Operators to initial and date each step.
In-Process Controls:
- Detailed instructions for in-process testing and analysis.
- Acceptance criteria for each test.
- Space for recording test results, including:
- Test Method Reference
- Date and Time of Testing
- Results
- Operator Initials and Date
Packaging and Labeling:
- Detailed instructions for the packaging and labeling of the finished product.
- Specification of packaging materials, including:
- Type of Container
- Size
- Label Information (e.g., Lot number, expiration date)
- Inspection Procedures
Yield Calculations:
- Instructions for calculating actual yield at each stage of the process.
- Acceptance Criteria for Yield
Deviation Reporting:
- Space for documenting any deviations from the approved manufacturing process.
- Instructions for reporting deviations, including:
- Description of the deviation
- Root cause analysis
- Corrective and preventative actions taken.
Review and Approval:
- Space for the final review and approval of the MBR.
- Signatures and dates of authorized personnel.
Best Practices for Creating and Maintaining Your MBR Template
- Clarity and Precision: Use clear, concise language to avoid ambiguity. Instructions should be unambiguous and easy to follow.
- Standardization: Develop a standardized MBR template for all products to ensure consistency and ease of use.
- Regular Updates: Review and update the MBR regularly to reflect changes in processes, equipment, and regulations.
- Training: Ensure all personnel involved in manufacturing are adequately trained on the MBR and its use.
- Electronic Systems: Consider using electronic batch records (EBRs) for enhanced efficiency, traceability, and data integrity.
- Validation: Validate the MBR and the manufacturing process to ensure the product meets the required specifications.
- Control Changes: Implement change control procedures to manage and document any modifications to the MBR.
- Good Documentation Practices (GDP): Adhere to GDP principles, including:
- Writing legibly
- Using indelible ink
- Correcting errors properly (single line through, initialed, and dated)
- Avoiding blank spaces
The Benefits of a Well-Designed MBR Template
Implementing a well-crafted MBR template offers numerous benefits:
- Ensured Product Quality: Consistent adherence to the MBR leads to consistent product quality, reducing the risk of defects and recalls.
- Regulatory Compliance: Compliance with regulatory requirements (e.g., FDA, EMA) is significantly easier with a robust MBR system.
- Improved Efficiency: Standardized procedures and clear instructions streamline the manufacturing process, reducing errors and saving time.
- Enhanced Traceability: The MBR provides a complete audit trail, allowing for full traceability of materials, processes, and personnel.
- Reduced Risk: Minimizes the risk of manufacturing errors, deviations, and product failures.
- Facilitated Investigation: Streamlines investigations in case of deviations or product complaints.
Conclusion: Mastering the Batch Record
A well-designed Master Batch Record template is essential for any manufacturing operation striving for quality, compliance, and efficiency. By incorporating the key components and best practices outlined in this guide, you can create an MBR template that serves as the foundation for consistent, reliable, and compliant product manufacturing. Continuous improvement and adaptation to evolving regulations are crucial for maintaining a robust and effective MBR system.
Frequently Asked Questions (FAQs)
1. What is the difference between a Master Batch Record and a Batch Record?
The Master Batch Record (MBR) is the template or blueprint. It contains the detailed instructions for manufacturing a product. The Batch Record (also known as executed batch record or batch production record) is the completed record of a specific batch, with actual data and signatures entered during the manufacturing process, based on the Master Batch Record.
2. Who is responsible for creating and approving the Master Batch Record?
The responsibility for creating and approving the MBR typically lies with the Quality Assurance (QA) department, often in collaboration with the manufacturing and research and development (R&D) departments. The approval process must be completed by authorized personnel, ensuring that the MBR meets all regulatory requirements and is scientifically sound.
3. How often should the Master Batch Record be reviewed and updated?
The MBR should be reviewed and updated regularly, at a minimum annually, or whenever there is a change to the manufacturing process, equipment, or raw materials. This ensures that the MBR remains accurate, current, and compliant with regulatory requirements. The frequency of review and updates might be dictated by internal SOPs or regulatory requirements.
4. What are some common errors found in Master Batch Records?
Some common errors include:
- Missing or incomplete information.
- Ambiguous or unclear instructions.
- Failure to follow established procedures.
- Incorrect or missing data entries.
- Lack of proper review and approval.
- Failure to document deviations.