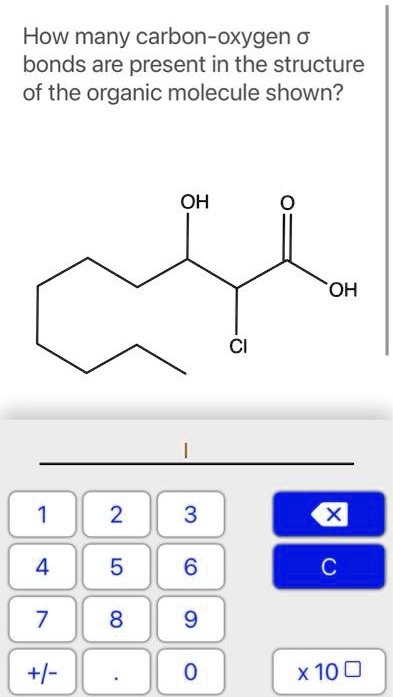
Carbon-Oxygen Bonds: The Cornerstone of Organic Chemistry
Organic chemistry, the study of carbon-containing compounds, forms the backbone of life as we know it. From the sugars that fuel our bodies to the plastics that shape our world, organic molecules are everywhere. At the heart of this vast and complex field lies a fundamental concept: the carbon-oxygen bond (C-O bond). Understanding this bond is absolutely crucial for anyone seeking to grasp the principles of organic chemistry. This article will delve into the significance of C-O bonds, exploring their characteristics, reactivity, and their profound impact on the structure and function of organic molecules.
The Importance of Carbon-Oxygen Bonds
The C-O bond is not just a simple connection; it’s a gateway to a diverse range of functional groups, the specific arrangements of atoms within a molecule that dictate its reactivity and properties. These functional groups are the building blocks of organic chemistry, and the presence of a carbon-oxygen bond is central to several key ones, including:
- Alcohols (R-OH): Characterized by a hydroxyl group (-OH) bonded to a carbon atom.
- Ethers (R-O-R’): Contain an oxygen atom bridging two carbon groups.
- Aldehydes (R-CHO): Featuring a carbonyl group (C=O) at the end of a carbon chain, with a hydrogen atom attached to the carbonyl carbon.
- Ketones (R-CO-R’): Possessing a carbonyl group within a carbon chain, bonded to two other carbon groups.
- Carboxylic Acids (R-COOH): Containing a carboxyl group (-COOH), which is a carbonyl group attached to a hydroxyl group.
- Esters (R-COOR’): Formed by the reaction of a carboxylic acid with an alcohol.
- Epoxides: Cyclic ethers with a three-membered ring.
These functional groups, all featuring C-O bonds, exhibit unique chemical behaviors, allowing for a vast array of chemical transformations and the creation of countless organic compounds.
Properties of the Carbon-Oxygen Bond
The C-O bond possesses several key properties that influence its behavior and reactivity:
- Polarity: Oxygen is significantly more electronegative than carbon. This means that the shared electrons in the C-O bond are pulled closer to the oxygen atom, creating a dipole moment. The oxygen atom acquires a partial negative charge (δ-), and the carbon atom acquires a partial positive charge (δ+). This polarity is crucial for understanding the reactivity of molecules containing C-O bonds.
- Bond Strength: The C-O bond is relatively strong, requiring a significant amount of energy to break. This stability contributes to the prevalence and importance of carbon-oxygen bonds in organic molecules.
- Bond Length: The C-O bond is shorter than a single carbon-carbon bond. This is due to the smaller atomic radius of oxygen and the greater attraction between the bonded atoms.
Reactivity and Reactions Involving Carbon-Oxygen Bonds
The polarity of the C-O bond makes the carbon atom susceptible to attack by nucleophiles (electron-rich species), while the oxygen atom can be attacked by electrophiles (electron-deficient species). This characteristic facilitates a wide range of important chemical reactions, including:
- Nucleophilic Addition: Where a nucleophile attacks the electrophilic carbon of a carbonyl group (C=O), common in reactions of aldehydes and ketones.
- Nucleophilic Acyl Substitution: Where a nucleophile replaces a leaving group attached to a carbonyl carbon, typical of reactions involving carboxylic acid derivatives.
- Esterification: The reaction of a carboxylic acid with an alcohol to form an ester.
- Hydrolysis: The breaking of a C-O bond by reaction with water, often used to break down esters and other compounds.
Understanding these reaction mechanisms is fundamental to predicting and controlling the behavior of organic molecules in various chemical transformations.
The Role of C-O Bonds in Biological Systems
Carbon-oxygen bonds are absolutely essential in the intricate chemistry of life:
- Carbohydrates: Sugars, starches, and cellulose, all are built on a foundation of C-O bonds within their ring and chain structures. They provide energy and structural support in living organisms.
- Proteins: The peptide bonds that link amino acids together, forming the backbone of proteins, are stabilized by C-O bonds in the carbonyl group of the amino acid residues.
- Lipids: Fats, oils, and phospholipids, essential for cell structure and energy storage, contain ester linkages (C-O bonds).
- DNA and RNA: The sugar-phosphate backbone of these genetic materials relies on C-O bonds to connect the sugar molecules.
Conclusion
The carbon-oxygen bond is far more than just a chemical connection; it’s a fundamental pillar of organic chemistry. Its polarity, strength, and the functional groups it enables are the building blocks for understanding the structure, reactivity, and function of countless organic molecules. From the smallest alcohol molecule to the most complex biomolecules, C-O bonds play a critical role. A strong understanding of C-O bonds is, therefore, a prerequisite for mastering organic chemistry and its applications in fields ranging from medicine and materials science to agriculture and environmental science.
Frequently Asked Questions (FAQs)
Why is the C-O bond polar? The C-O bond is polar because oxygen is more electronegative than carbon. This means oxygen attracts the shared electrons in the bond more strongly, leading to a partial negative charge on the oxygen and a partial positive charge on the carbon.
What are some key functional groups that contain C-O bonds? Key functional groups containing C-O bonds include alcohols, ethers, aldehydes, ketones, carboxylic acids, esters, and epoxides.
How does the polarity of the C-O bond affect its reactivity? The polarity makes the carbon atom susceptible to nucleophilic attack, while the oxygen atom can be attacked by electrophiles. This characteristic is a crucial component of understanding how organic molecules react.
Where are C-O bonds found in biological systems? C-O bonds are found in carbohydrates, proteins, lipids, and the sugar-phosphate backbone of DNA and RNA. They are essential for the structure and function of these biomolecules.
What are the main types of reactions involving C-O bonds? Main reactions involving C-O bonds include nucleophilic addition, nucleophilic acyl substitution, esterification, and hydrolysis.